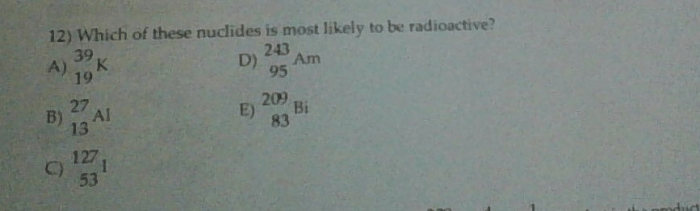Which of these nuclides is most likely to be radioactive? This question delves into the fascinating realm of nuclear physics, where the stability and decay of atomic nuclei hold the key to understanding the fundamental forces that govern our universe.
Join us as we embark on a journey to explore the intricacies of isotope stability, radioactive decay processes, and the methods used to detect and measure radioactivity, unraveling the mysteries that lie at the heart of matter.
As we delve deeper into this topic, we will encounter concepts such as neutron-to-proton ratio, alpha, beta, and gamma decay, half-life, and decay rates. Along the way, we will uncover the factors that influence the stability of isotopes and the mechanisms behind their radioactive transformations.
By examining real-world examples and engaging in thought-provoking discussions, we aim to shed light on the fascinating world of nuclear science and its profound implications for our understanding of the universe.
Isotope Stability: Which Of These Nuclides Is Most Likely To Be Radioactive

Isotope stability refers to the tendency of an isotope to remain unchanged over time. Stable isotopes do not undergo radioactive decay, while unstable isotopes do. The stability of an isotope is determined by its neutron-to-proton ratio.
Isotopes with a neutron-to-proton ratio close to 1 are generally stable. For example, carbon-12 (6 protons, 6 neutrons) is a stable isotope, while carbon-14 (6 protons, 8 neutrons) is an unstable isotope.
Radioactive Decay Processes, Which of these nuclides is most likely to be radioactive
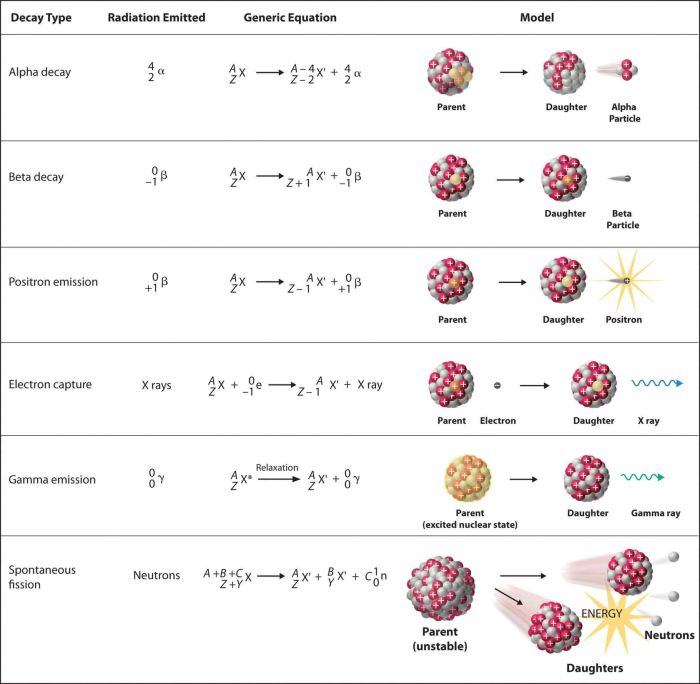
Radioactive decay is the process by which an unstable isotope transforms into a more stable isotope. There are three main types of radioactive decay: alpha decay, beta decay, and gamma decay.
- Alpha decayis the emission of an alpha particle, which is a helium nucleus (2 protons, 2 neutrons). Alpha decay occurs when the nucleus of an atom is too large and unstable.
- Beta decayis the emission of a beta particle, which is an electron or a positron. Beta decay occurs when the nucleus of an atom has too many or too few neutrons.
- Gamma decayis the emission of a gamma ray, which is a high-energy photon. Gamma decay occurs when the nucleus of an atom is in an excited state.
Half-Life and Decay Rates
Half-life is the amount of time it takes for half of the atoms in a sample of a radioactive isotope to decay. Half-life is a constant for a given isotope and is independent of the amount of the isotope present.
The decay rate of a radioactive isotope is the number of atoms that decay per unit time. The decay rate is proportional to the half-life of the isotope.
Detection and Measurement of Radioactivity
Radioactivity can be detected and measured using a variety of devices, including Geiger counters and scintillation detectors.
- Geiger countersdetect radioactivity by measuring the ionization produced by radiation.
- Scintillation detectorsdetect radioactivity by measuring the light produced when radiation interacts with a scintillator material.
FAQ Corner
What is the difference between a stable and an unstable isotope?
Stable isotopes have a balanced neutron-to-proton ratio, making them resistant to radioactive decay. Unstable isotopes, on the other hand, have an imbalance in their neutron-to-proton ratio, leading to spontaneous radioactive decay.
How does radioactive decay occur?
Radioactive decay is a process by which an unstable isotope transforms into a more stable form, releasing energy in the form of particles or electromagnetic radiation. Common types of radioactive decay include alpha decay, beta decay, and gamma decay.
What is half-life?
Half-life is the time it takes for half of the radioactive atoms in a sample to decay. It is a characteristic property of each radioactive isotope and is used to determine the rate of radioactive decay.