Delve into the fascinating realm of chemistry with our comprehensive periodic table worksheet with answer key. This invaluable resource empowers you to decipher the secrets of the periodic table, unlocking a deeper understanding of the elements that shape our world.
Embark on an educational journey that unravels the organization, structure, and history of the periodic table. Explore the diverse elements that reside within its confines, delving into their properties, characteristics, and applications.
Periodic Table Overview
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties. It is generally accepted that the modern periodic table was first published by Dmitri Mendeleev in 1869, although several other scientists had developed similar tables prior to this.
The periodic table is organized into 18 vertical columns, called groups, and 7 horizontal rows, called periods. The groups are numbered 1-18 from left to right, and the periods are numbered 1-7 from top to bottom.
Elements in the same group have similar chemical properties, while elements in the same period have similar atomic radii and ionization energies.
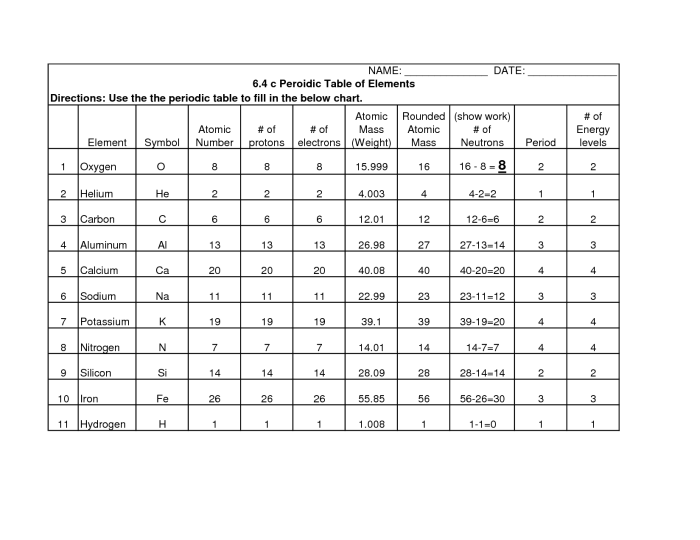
Elements on the Periodic Table, Periodic table worksheet with answer key
The periodic table contains 118 elements, which are the building blocks of all matter. Elements are classified as metals, nonmetals, or metalloids based on their physical and chemical properties.
- Metals are shiny, malleable, and ductile. They are good conductors of heat and electricity.
- Nonmetals are dull, brittle, and poor conductors of heat and electricity.
- Metalloids have properties of both metals and nonmetals.
The most common element in the universe is hydrogen. The most common element in the Earth’s crust is oxygen.
Groups and Periods
The elements in the periodic table are arranged into groups and periods based on their atomic number and electron configurations.
Groups are vertical columns in the periodic table. Elements in the same group have the same number of valence electrons, which are the electrons in the outermost shell of an atom. Valence electrons determine the chemical properties of an element.
Periods are horizontal rows in the periodic table. Elements in the same period have the same number of electron shells.
Trends and Patterns
There are several trends and patterns in the periodic table. These trends and patterns can be used to predict the properties of an element.
- As you move from left to right across a period, the elements become more nonmetallic.
- As you move down a group, the elements become more metallic.
- The elements in the noble gas group are all very stable and do not react with other elements.
Applications of the Periodic Table
The periodic table is a valuable tool for scientists and engineers. It is used in a wide variety of applications, including:
- Predicting the properties of new elements
- Developing new materials
- Understanding chemical reactions
- Classifying elements
- Teaching chemistry
Common Queries: Periodic Table Worksheet With Answer Key
What is the purpose of a periodic table?
A periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties.
How are elements classified on the periodic table?
Elements are classified into groups (vertical columns) and periods (horizontal rows) based on their similarities in chemical properties and electron configurations.
What information can be found in an answer key for a periodic table worksheet?
An answer key for a periodic table worksheet typically provides detailed explanations for each answer, including references or sources for further exploration.